How do D-orbital nano oxide (DNO) catalysts work in air purification?
11 February 2026
Key points
-
D-orbital nano oxide (DNO) describes a family of transition-metal oxide catalysts used to break down some indoor air pollutants at a solid surface.
-
In a catalytic system, pollutants are captured near the surface and then oxidised into more stable end products.
-
DNO catalysts are commonly used alongside mechanical filtration (e.g. HEPA) so that particles are physically captured while certain gases and bioaerosols can be treated at or near the surface.
-
A practical advantage of surface-confined catalysis is that it aims to avoid the “bulk-air chemistry” that can contribute to secondary air pollution (for example, ozone or reactive by-products in the occupied room).
-
The best way to evaluate any catalytic air-cleaning claim is to look for independent test methods and whether tests checked not only pollutant removal but also potential by-products.
D-orbital nano oxide (DNO) catalysts are a way to remove pollutants without relying on reactive chemistry occurring throughout the room air. Instead, the chemistry is intended to occur on the catalyst surface, where pollutants have been trapped.
This article explains, in simple terms, what DNO catalysts are, how they operate in an air-cleaning system, and what to look for when interpreting the evidence.
For a broader overview of technology families, see How different indoor air purification technologies work.
What does “D-orbital nano oxide” mean in practice?
“D-orbital” points to the fact that many useful catalysts are based on transition metals (elements in the d-block of the periodic table). Their oxides can support surface reactions because they can change oxidation state and form active oxygen species under the right conditions.
“Nano oxide” indicates that these are metal oxide materials engineered to present a high surface area and high reactivity at their surfaces. In air cleaning, the surface is the key: the catalyst’s job is to create a location where pollutants can be adsorbed and then chemically transformed.
The practical consequence is that a DNO system is not “just a filter”. It is a surface reactor integrated into an airflow device.
What are DNO catalysts made of?
In the published DNO literature, the catalysts are described as combinations of transition-metal oxides (for example, oxides of manganese, zinc, and silver) supported on high-surface-area substrates.
Two design ideas matter more than the exact formulation:
-
High surface area support
The support provides lots of surface for contact between the air and catalyst. In some designs, supports are chosen to be relatively hydrophilic (water-attracting), which can help trap more polar pollutants and biological material at the surface. -
Active metal oxide sites
The metal oxide sites provide the chemistry. Under operating conditions, they can activate oxygen and support oxidation reactions of adsorbed compounds.
In other words, the support helps pollutants arrive and stay on the surface long enough, and the active sites do the chemical work.
How does a catalytic surface remove VOCs and odours?
A useful way to think about VOC removal in catalysis is as a sequence:
-
Transport to the surface
Airflow brings VOC molecules close to the catalyst. -
Adsorption/trapping
VOCs and other gases can adhere to the surface (especially if the support has suitable surface chemistry and porosity). -
Surface oxidation
Oxygen in the air can be activated at the catalyst surface, forming reactive oxygen species that remain surface-bound. -
Conversion to stable products
The surface reaction breaks down larger, more reactive organic molecules into more stable end products. For many organic gases, the “fully oxidised” end state is carbon dioxide and water, though real indoor air chemistry can be more complex depending on the pollutant mix.
In an engineering sense, this is similar to catalytic oxidation used in other applications: the catalyst accelerates reactions that would otherwise be too slow at room temperature.
How can a catalyst affect bioaerosols?
Bioaerosols (such as bacteria, mould fragments, and virus-containing aerosols) behave differently from gases:
-
They are carried by particles or droplets.
-
They can deposit on filters and surfaces.
-
Their “removal” may involve capture, dehydration, damage to biological structures, or chemical inactivation at a surface.
In a combined system (filtration + catalyst), the usual intent is:
-
Mechanical filter media captures the particle or droplet (physical removal from the air stream).
-
The catalytic layer provides a surface environment that can contribute to deactivation and breakdown of organic material at or near where the material has been captured.
This matters for two reasons:
-
It can add an inactivation step rather than simply storing captured biological material in a filter.
-
It can reduce concerns about secondary release (re-entrainment of viable material) during long operation or handling, because the goal is not only to trap but also to deactivate.
Why does DNO focus on “surface-confined” chemistry?
A recurring theme in modern indoor air cleaning is the difference between:
-
Bulk-air chemistry: reactive species are created in the room air (or leak into it), where they can react with whatever mixture of VOCs happens to be present.
-
Surface-confined chemistry: reactive species are generated and used at a surface, aiming to keep the high-energy chemistry away from the occupied air volume.
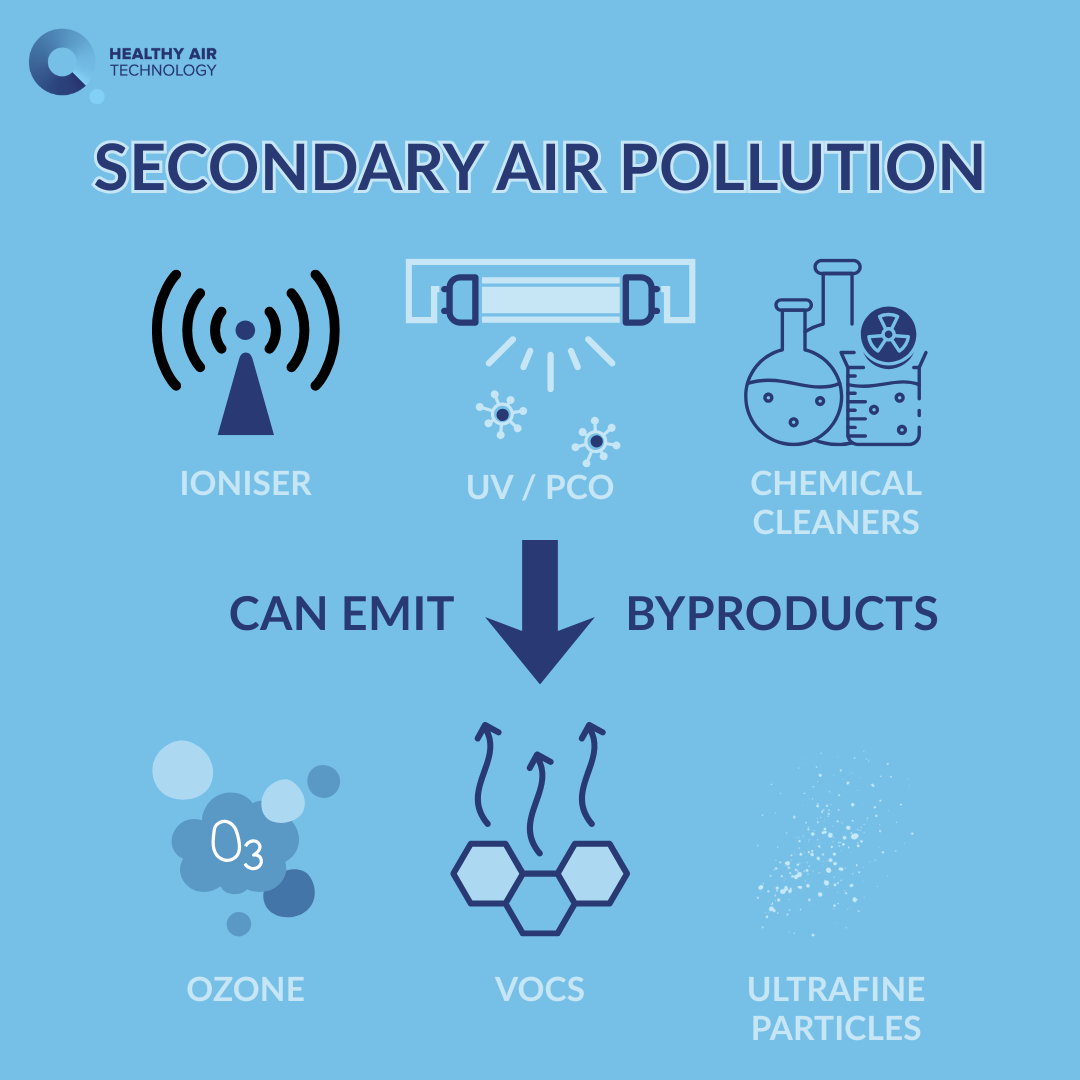
This matters because uncontrolled oxidation chemistry in occupied air can create by-products. In the Knowledge Hub, we describe that risk as secondary air pollution—pollution that is formed or re-released as a side effect of air cleaning.
A surface-confined catalytic approach is intended to reduce that risk by keeping reactive intermediates bound to a surface rather than circulating through the room.
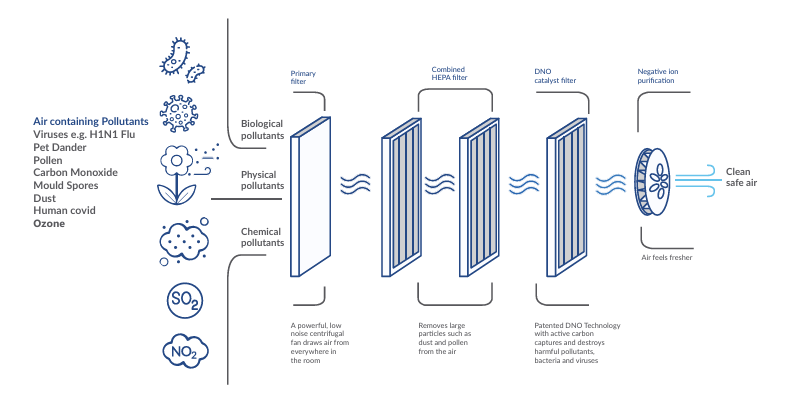
How do DNO catalysts relate to filtration stages?
A practical DNO-based air purification system is usually described as multi-stage:
-
Pre-filtration: reduces large dust and fibres, protecting downstream stages.
-
High-efficiency particle filtration (often described as HEPA-class media): captures fine particles and bioaerosols.
-
Catalytic stage (DNO): treats gases/VOCs and provides a surface for oxidative breakdown and inactivation near captured material.
-
(Sometimes) additional stages, such as carbon media, depending on configuration.
This layered design matters because indoor air is a mixture problem: particles, gases, and bioaerosols typically coexist. No single stage is optimal for all categories.
Does a DNO catalytic system generate ozone or other by-products?
This is exactly the right question to ask of any “active” air cleaning technology.
The best answer is never “trust the mechanism”, but rather:
-
What was measured during operation?
-
Under what conditions (airflow, humidity, pollutant mix, long run time)?
-
Were likely by-products (such as ozone) explicitly checked?
Catalytic systems are commonly presented as a route to avoid bulk-air oxidant release. However, the only robust way to confirm that in practice is through test data that includes by-product monitoring.
This is why the Knowledge Hub separates:
-
Primary pollutant removal (what you want to reduce), from
-
Secondary pollution risk (what you might unintentionally create).
How are DNO catalysts tested and reported?
Catalytic air-cleaning claims are typically supported by two types of evidence:
Controlled tests (chambers/rigs)
These tests use a defined pollutant mixture and measure concentrations upstream and downstream of the system over time. They are useful for:
-
VOC removal rates (e.g. formaldehyde or representative VOCs)
-
Checking whether any by-products appear
-
Comparing performance across conditions (humidity, flow rate)
Real environment studies (field use)
Field studies measure real indoor conditions (particles, TVOCs, sometimes specific gases, sometimes microbiological sampling) before/during/after device operation. They are useful for:
-
Showing how performance translates into occupied spaces
-
Demonstrating stability over time
-
Showing what happens when devices are switched off or removed
Both matter: controlled tests help explain “what the system can do”, while field studies help explain “what it does do” in ordinary buildings.
If you want to read the underlying technical discussion, you can link directly to the published DNO paper/white paper from this page.
What should readers look for when evaluating DNO (or any catalytic) claims?
A practical checklist that stays technology-neutral:
-
Which pollutants were tested?
Particle removal data does not automatically translate to VOC removal, and vice versa. -
Was the test method appropriate?
Look for clarity about chamber size, airflow, starting concentrations, and run time. -
Were by-products measured?
Especially relevant if a technology uses “active” chemistry. -
How is maintenance handled?
Filters load; sorbents saturate; performance can drift. The system is only as good as its operation and maintenance plan. -
Is there evidence in realistic environments?
Results from occupied buildings help ground the discussion.
This mindset is aligned with the Knowledge Hub’s overall theme: indoor air quality is measurable and manageable, but you need to match the mechanism to the pollutant and watch for unintended side effects.
Summary
D-orbital nano oxide (DNO) catalysts are a surface-based approach to indoor air cleaning: pollutants are captured at or near a catalytic surface and then oxidised toward stable products. In a multi-stage system, the catalyst is typically paired with high-efficiency filtration so that particles are physically removed while certain gases and bioaerosols can be treated at the surface.
When comparing catalytic technologies, the most useful questions are practical: what was tested, what was measured (including by-products), and how does the system behave over time in real buildings.
Latest News

How do D-orbital nano oxide (DNO) catalysts work in air purification?
Key points D-orbital nano oxide (DNO) describes a family of transition-metal oxide catalysts used to break down some…

How do different indoor air purification technologies actually work?
Key points “Air purification” is not one technology: different systems target particles, gases/VOCs, and bioaerosols in different ways….
What does “secondary air pollution” actually mean indoors?
Key points Secondary air pollution refers to new pollutants or re-released contaminants created as a side effect of…

